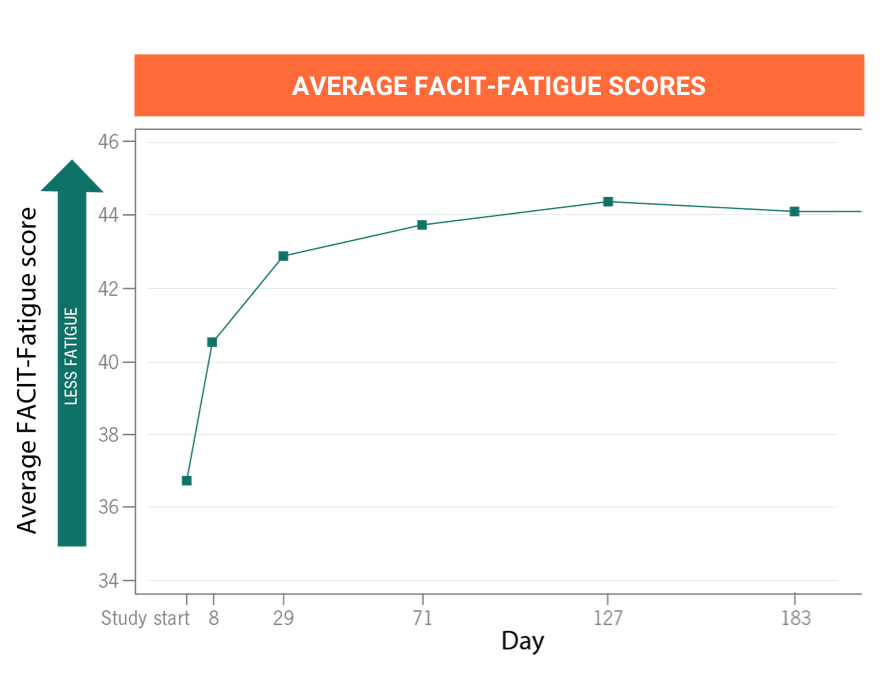
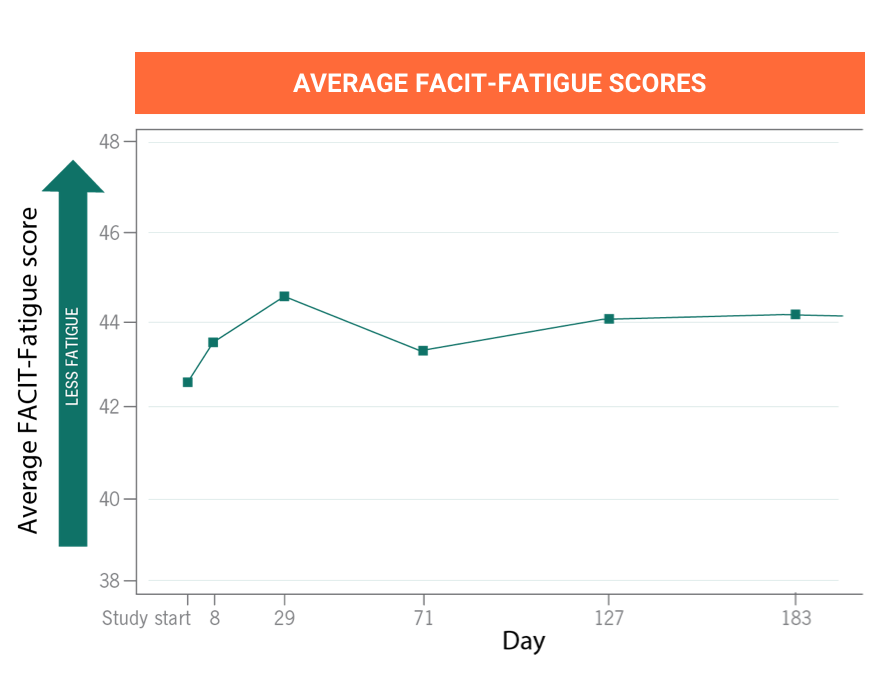
ULTOMIRIS provides immediate, complete, and sustained C5 control for up to 8 weeksa
Many clinical trials have established the short- and long-termb efficacy for starting or switching to ULTOMIRIS in people diagnosed with PNH.
aTwo weeks after the intravenous loading dose, ULTOMIRIS is infused intravenously every 8 weeks for most people and every 4 weeks for children weighing less than 44 pounds (20 kilograms).
bThe primary evaluation period of the clinical trials was 26 weeks and the extension period of the trials had a range of 3 to 5 years.
Getting started on ULTOMIRIS
While learning about the signs and symptoms associated with living with PNH can be unsettling, you should also know that this condition is manageable with ULTOMIRIS.
STARTING ULTOMIRISAlexion OneSourceTM Copay Program
Pay as low as
$0
In out-of-pocket costs
if you're eligiblea,b
See if you're eligible
• Valid only for individuals with commercial insurance who have a valid prescription for a US FDA-approved indication of ULTOMIRIS
• Not valid for individuals covered by government insurance programs or other federal or state programs (including any state prescription drug assistance programs)
aBased on typical commercial patients out-of-pocket deductible limits.
bAdditional teams and conditions apply. Please contact OneSource with additional questions.
cIncludes Medicaid, Medicare (including Medicare Prat D), Medicare Advantage Plans, Medigap, Veterans Affairs, Department of Defense, or TRICARE. Patients reading in Massachusetts or Rhode Island are eligible for assistance with medication costs but are not eligible for assistance with inclusion costs.
What is the most important information I should know about ULTOMIRIS?
ULTOMIRIS is a medicine that affects your immune system and can lower the ability of your immune system to fight infections.
- ULTOMIRIS increases your chance of getting serious meningococcal infections that may quickly become life-threatening or cause death if not recognized and treated early.
- You must complete or update meningococcal vaccine(s) at least 2 weeks before your first dose of ULTOMIRIS.
- If you have not completed your meningococcal vaccines and ULTOMIRIS must be started right away, you should receive the required vaccine(s) as soon as possible.
- If you have not been vaccinated and ULTOMIRIS must be started right away, you should also receive antibiotics for as long as your healthcare provider tells you.
- If you had a meningococcal vaccine in the past, you might need additional vaccines before starting ULTOMIRIS. Your healthcare provider will decide if you need additional meningococcal vaccines.
- Meningococcal vaccines do not prevent all meningococcal infections. Call your healthcare provider or get emergency medical care right away if you get any of these signs and symptoms of a meningococcal infection: fever, fever with high heart rate, headache and fever, confusion, body aches with flu-like symptoms, fever and a rash, headache with nausea or vomiting, headache with a stiff neck or stiff back, or eyes sensitive to light.
Your healthcare provider will give you a Patient Safety Card about the risk of serious meningococcal infection. Carry it with you at all times during treatment and for 8 months after your last ULTOMIRIS dose. Your risk of meningococcal infection may continue for several months after your last dose of ULTOMIRIS. It is important to show this card to any healthcare provider who treats you. This will help them diagnose and treat you quickly.
ULTOMIRIS is only available through a program called the ULTOMIRIS Risk Evaluation and Mitigation Strategy (REMS). Before you can receive ULTOMIRIS, your healthcare provider must: enroll in the REMS program; counsel you about the risk of serious meningococcal infections; give you information about the signs and symptoms of serious meningococcal infection; make sure that you are vaccinated against serious infections caused by meningococcal bacteria, and that you receive antibiotics if you need to start ULTOMIRIS right away and are not up to date on your vaccines; give you a Patient Safety Card about your risk of meningococcal infection.
ULTOMIRIS may also increase the risk of other types of serious infections, including Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria gonorrhoeae. Your child should receive vaccines against Streptococcus pneumoniae and Haemophilus influenzae type b (Hib) if treated with ULTOMIRIS. Certain people may be at risk of serious infections with gonorrhea.
Who should not receive ULTOMIRIS?
Do not receive ULTOMIRIS if you have a serious meningococcal infection when you are starting ULTOMIRIS.
Before you receive ULTOMIRIS, tell your healthcare provider about all of your medical conditions, including if you: have an infection or fever, are pregnant or plan to become pregnant, and are breastfeeding or plan to breastfeed. It is not known if ULTOMIRIS will harm your unborn baby or if it passes into your breast milk. You should not breastfeed during treatment and for 8 months after your final dose of ULTOMIRIS.
Tell your healthcare provider about all the vaccines you receive and medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements which could affect your treatment.
If you have PNH and you stop receiving ULTOMIRIS, your healthcare provider will need to monitor you closely for at least 16 weeks after you stop ULTOMIRIS. Stopping ULTOMIRIS may cause breakdown of your red blood cells due to PNH. Symptoms or problems that can happen due to red blood cell breakdown include: drop in your red blood cell count, tiredness, blood in your urine, stomach-area (abdomen) pain, shortness of breath, blood clots, trouble swallowing, and erectile dysfunction (ED) in males.
What are the possible side effects of ULTOMIRIS?
ULTOMIRIS can cause serious side effects including infusion-related reactions. Symptoms of an infusion-related reaction with ULTOMIRIS may include lower back pain, tiredness, feeling faint, discomfort in your arms or legs, bad taste, or drowsiness. Stop treatment of ULTOMIRIS and tell your healthcare provider or nurse right away if you develop these symptoms, or any other symptoms during your ULTOMIRIS infusion that may mean you are having a serious infusion reaction, including: chest pain, trouble breathing or shortness of breath, swelling of your face, tongue, or throat, and feel faint or pass out.
The most common side effects of ULTOMIRIS in people treated for PNH are upper respiratory tract infection and headache.
Tell your healthcare provider about any side effect that bothers you or that does not go away. These are not all the possible side effects of ULTOMIRIS. For more information, ask your healthcare provider or pharmacist. Call your healthcare provider right away if you miss an ULTOMIRIS infusion or for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
INDICATION
What is ULTOMIRIS?
ULTOMIRIS is a prescription medicine used to treat adults and children 1 month of age and older with a disease called Paroxysmal Nocturnal Hemoglobinuria (PNH).
It is not known if ULTOMIRIS is safe and effective in children younger than 1 month of age.
Please see the full Prescribing Information and Medication Guide for ULTOMIRIS, including Boxed WARNING regarding serious meningococcal infections.